>>> 9-1-1, we have an emergency…
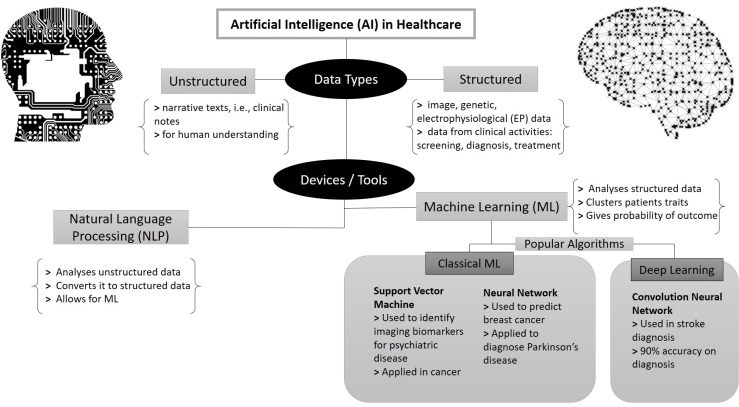
AI is not just appearing in our homes with the use of Amazon’s Alexa nor just helping us in transportation with self-driving Tesla cars, but also in healthcare. As we shift from all manual based medicine to an AI-human interactive healthcare, the question in this title is one of the many that needs to be addressed (See Blog Post)
I would like to discuss the view of a fellow blogger, Shailin Thomas, in his Harvard Law Bill of Health blog. He argues that since the algorithm has a higher accuracy rate than the average doctor and going with the suggestion of the algorithm is the statistically best option to follow, then it “seems wrong to place blame on the physician”.
He then focuses on the fact that shifting this blame away from the doctor would lead to a decrease in the exercise of medical malpractice laws , which protect patients by providing a way to police their doctors final diagnosis, and gives two reasons why this could be a good news. First is that strict malpractice liability laws do not necessarily mean a better result for the patient as suggested by research done at Northwestern University reported by FoxNews. Second, that taking malpractice liability away from the physician would decrease the overspending in healthcare resulting from the practice of defensive medicine (ordering more diagnostic tests to avoid potential lawsuits).
Although he certainly makes good use of potential positive outcome of lessening medical malpractice laws on physicians, I strongly disagree that tempering with the serious responsibility of an AI-based diagnostic mistake is the way to effect this change.
The doctor is the healthcare professional, not the AI. Regardless of the use of the AI algorithm, at the end of the day, the algorithm continues to remain a tool for the physician to improve the accuracy and delivery of her diagnosis. As pediatric Rahul Parikh mentions in his MIT technology review, “AI can’t replace doctors. But it can make them better”. After all, AI is not replacing their jobs, but changing them. Take, for example, if you require a certain complex program to do an aspect of your job you had to do manually before. At first you are sceptic of its use, but as time passes, you become familiar with the program. So much that there comes a point where you decide to fully trust it, bypassing any double inspections. But then you make a mistake. You realize the program made it without crossing your mind to second guess. Will your employer fire you, or the program? In healthcare there is seldom firing, usually there is death.
“There is currently no safety body that can oversee or accredit any of these medical AI technologies, and we generally don’t teach people to be safe users of the technology. We keep on unleashing new genies and we need to keep ahead of it.”
-Enrico Coiera, Director of the Centre for Health Informatics at Macquarie University
The healthcare industry is already good at failing to prevent mistakes. In 2016, medical errors were the 3rd leading cause of death in the U.S only, where only heart disease and cancer exceeded it. There have been many commentators comparing AI like a black box, as it is nearly impossible to know how the deep learning algorithms come up with their conclusions. But healthcare industries can also be classified as a black box, as Matthew Syed references in his Black Box Thinking book, where everyone trust doctors and hospitals and yet they have let more people die each year than in traffic accidents while the process is overall sorrowful but accepting, with limited post hoc oversight .
| Industry | Deaths (2016) |
| Aviation | 325 |
| Traffic accidents | 40,000 |
| Healthcare industry (preventable) | 250, 000 |
If the physician will not be responsible, who will? The first option would be to hold the AI responsible. However, the AI is inanimate where the affected patient would have no compensation and will end extremely unsatisfied. A second option would be to shift the blame to the developers. This might be difficult as the software can be accurate on its initial design and implementation, for example, IBM Watson in clinical medicine where it was designed to compete in Jeopardy. This would also decrease interest in AI development. A third option would be to hold the organization behind the AI responsible. However, if the AI does not have design failures it would be hard for this to work, like holding Tesla responsible for an accident done by a Tesla car user.
To further develop AI implementation in healthcare, the question of responsibility needs to be addressed. But in your case, who do you think should be held responsible?





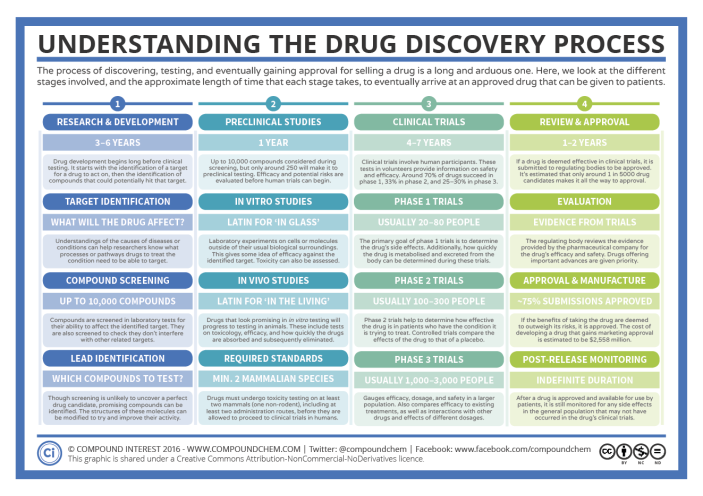 In this post, I will be focusing on the drug discovery ( research & development) stage, which focuses on identifying the perfect drug candidate from many molecules able to have the desired therapeutic effect on a biological target of interest ( i.e., a protein).
In this post, I will be focusing on the drug discovery ( research & development) stage, which focuses on identifying the perfect drug candidate from many molecules able to have the desired therapeutic effect on a biological target of interest ( i.e., a protein).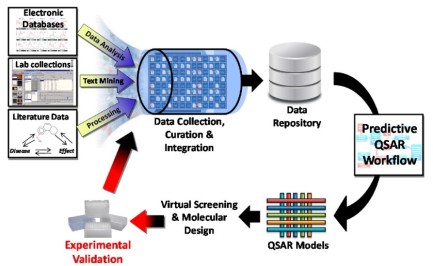 In its simplistic form, the measured activities of many small molecules against a single protein is obtained experimentally, then the
In its simplistic form, the measured activities of many small molecules against a single protein is obtained experimentally, then the